Cardiovascular Research
Cardioprotection
Cardiac repair
Cardiac regeneration
Gene therapy
Department of Medical Biology
Amsterdam University Medical Centers
The Netherlands
GLADKA
Research Group
We are on the quest to cure heart diseases
ATGAAGCAGCCGATCATGGCGGATGGCCCCCGGTGCAAGAGGCGCAAACAAGCCAATCCCAGGAGGAAAAACGTGGTGAACTATGACAATGTAGTGGACACAGGTTCTGAAACAGATGAGGAAGACAAGCTTCATATTGCTGAGGATGACGGTATTGCCAACCCTCTGGACCAGGAG
ATGAAGCAGCCGATCATGGCGGATGGCCCCCGGTGCAAGAGGCGCAAACAAGCCAATCCCAGGAGGAAAAACGTGGTGAACTATGACAATGTAGTGGACACAGGTTCTGAAACAGATGAGGAAGACAAGCTTCATATTGCTGAGGATGACGGTATTGCCAACCCTCTGGACCAGGAG
ATGAAGCAGCCGATCATGGCGGATGGCCCCCGGTGCAAGAGGCGCAAACAAGCCAATCCCAGGAGGAAAAACGTGGTGAACTATGACAATGTAGTGGACACAGGTTCTGAAACAGATGAGGAAGACAAGCTTCATATTGCTGAGGATGACGGTATTGCCAACCCTCTGGACCAGGAG
About
Our overarching purpose is to cure heart diseases, not simply as a scientific goal, but because heart diseases have profound implications for the quality of life.
To achieve our mission, we embark on a comprehensive journey through the intricate stages of heart failure. We delve into the molecular mechanisms that drive cardioprotection and cardiac repair by employing state-of-the-art technology, such as single-cell techniques and AAV-mediated gene therapies, to push the boundaries of what’s possible in cardiovascular research.
Our ultimate goal is to discover groundbreaking therapies for heart diseases. We look for therapies that provide medical advancements and hope for a healthier life for all those affected by this devastating condition.
Ischemic Heart Disease
Heart failure due to ischemic heart disease is a leading cause of morbidity and death worldwide. After an ischemic insult, patients develop a stiff fibrotic scar that hampers cardiac contractility, leading to HF. Since endogenous cardiac repair mechanisms of an adult mammalian heart are insufficient to repopulate lost myocardium and restore cardiac function, there is an urgent need to initiate cardioprotective mechanisms to minimize damage caused by ischemia and stimulate repair mechanisms in the already damaged heart.
Cardiac Repair
Cardiac repair is evidenced by increased cardiomyogenesis and angiogenesis, attenuated myocardial apoptosis and fibrosis, and improved ventricular function. We use state-of-the-art technologies to study cardiac repair mechanisms to discover new therapeutic targets.
Gene Therapy
We are using AAV-mediated gene transfer in vivo to test the therapeutic potential of newly identified factors. For this part, we closely collaborate with Prof. Mauro Giacca from Kings’ College in London and Dr Lorena Zentilin from Trieste.
Team

MONIKA GLADKA
Group leader
Monika is an Associate Professor and Principal Investigator at the Department of Medical Biology at Amsterdam University Medical Centers. She has 16 years of experience in molecular biology and animal studies. She did her PhD in the lab of Prof. de Windt, where she studied gene regulation during progression to heart failure. After that, she joined the lab of Prof. van Rooij where she focused on understanding the molecular mechanisms of cardiac regeneration.
Her current research focuses on understanding the molecular mechanisms that regulate cardiac repair, intending to identify new players to develop novel, improved gene therapies. She uses several state-of-the-art techniques, such as single-cell sequencing, enabling an in-depth mechanistic understanding of the biological processes in injured hearts. Beyond her scientific pursuits, she actively contributes to several cardiac societies, serving as a council member of the European section of the International Society of Heart Research (ISHR) and a nucleus member of the CARE working group from the European Society of Cardiology. Additionally, she serves as an associate editor at the Journal of Molecular Therapy.

Rocco Caliandro
PhD student
Rocco started his PhD in 2021 and is working on a project funded by the Dutch Heart Foundation. His research focuses on unraveling the intricate role played by the ZEB2 transcription factor and Zeb2 antisense long-noncoding RNA in regulating cardiac repair post-cardiac ischemia. Rocco employs diverse research models, including in vitro cultures, mouse models, and innovative AAV-mediated gene delivery techniques. Pushing the boundaries of scientific exploration, he is also pioneering cutting-edge technologies. These include the application of single nuclei RNA-sequencing in human cardiac tissue and the development of methodologies for culturing myocardial slices from large animals and humans. His research is well-received in the cardiovascular community, as he was selected multiple times to give an oral presentation during national and international conferences, including the Dutch-German Joint Meeting (DGJM) of Molecular Cardiology and the International Society of Heart Research (ISHR). Rocco recently won the 1st Poster Prize at the DCVA-NLHI Translational Cardiovascular Research Meeting in June 2024 in Utrecht.

alexandra giovou
PhD student
Alexandra started her PhD in 2021 and is part of the OUTREACH consortium funded by the Dutch Heart Foundation. The consortium focuses on congenital heart diseases and is led by several experts in the field, including Prof. Christoffels. Alexandra’s projects are guided by the knowledge of Prof. Christoffels in gene regulation and congenital heart disease while also benefiting from Dr. Gladka’s insights into cardiac regeneration and gene therapies. Her research focuses on unraveling the role of the TBX5 transcription factor in the context of postnatal heart regeneration. Her work goes beyond that as she explores other target genes that can stimulate cardiomyocyte proliferation and angiogenesis in congenital heart disease scenarios. Alexandra employs multiple tools and techniques, including genetic mouse models and cutting-edge AAV-mediated gene therapy in vitro and in vivo settings.

azra husetic
PhD student
In 2023, Azra started her PhD journey, becoming an integral part of a collaborative project between Leiden UMC and Amsterdam UMC, funded by the Rembrandt Institute. Her research revolves around the utilization of human models to investigate cardioprotection following heart injuries.
Azra’s work is conducted in two distinct settings. She employs fetal cardiac slices to assess the regenerative response, collaborating closely with the LUMC group led by Dr Smits. Simultaneously, she delves into the intricacies of adult cardiac slices to investigate injury mechanisms, a domain supervised by the AUMC group under the guidance of Dr Gladka. The overarching objective is to uncover novel targets that hold therapeutic potential in the heart, contributing to the advancement of cardiovascular medicine.

Merel Ligtermoet
PhD student
m.l.ligtermoet@amsterdamumc.nl
Merel started her PhD in September 2024 and is working on a collaborative project within the International Cardiovascular Research Partnership, funded by the British Heart Foundation (BHF), the German Centre for Cardiovascular Research (DZHK) and the Dutch Heart Foundation (DHF). Merel will study the intricate mechanisms underlying dilated cardiomyopathy (DCM) and leverage advanced technologies and cutting-edge edgy approaches to look for improved therapeutic strategies. She will use, among others, genetic mouse models of DCM, CRISPR-Cas9 technology, and AAV9-mediated gene transfer.
Merel is also our former Master’s student. She started her internship in October 2023 and collaborated closely with Rocco. Together, they were dedicated to unraveling the intriguing function of the long non-coding RNA Zeb2os within the context of cardiac ischemia.

Huiling Zhou
Animal Technician
Huiling started as an animal technician in October 2024. She is supporting animal work for multiple projects.

Ranim Mahmoud
Former HBO student
Ranim joins our group for an internship between September 2024 and January 2024. The title of her thesis is “Correlating the Expression of ZEB2, TMSB4, and PTMA to Endothelial Cells Proliferation in Human Cardiac Tissues”.

Francesca Sacchi
Former Visiting PhD student
Francesca started her PhD in 2021 under the guidance of Prof. Gabriele D’Uva, at the Alma Mater Studiorum – University of Bologna, Italy. Her research aims to identify potential therapeutic strategies to treat heart failure and improve the outcome of heart injuries. Francesca employs a diverse array of techniques for her research work, including manipulation of genetic murine models, the culture of primary cardiac cells, in vitro and ex vivo proliferation and differentiation assays by immunofluorescence and time-lapse imaging. She will be visiting our group between May and August 2024.

Roos Wensveen
Former MSc student
Roos did her internship between December 2022 and June 2023. The title of her thesis was “Investigating the protective role of ZEB2 and its potential regulatory mechanisms in oxidative stress response during cardiac repair.”

Dave ter Horst
Former HBO student
Dave did his internship between September 2022 and January 2023. The title of his thesis was “Investigating the molecular mechanisms of myocardial infarction.”
Technologies
Mouse model of cardiac ischemia
Transgenic mouse models
AAV-mediated gene therapy
Living myocardial slices cultures
Single-cell transcriptomics
Projects
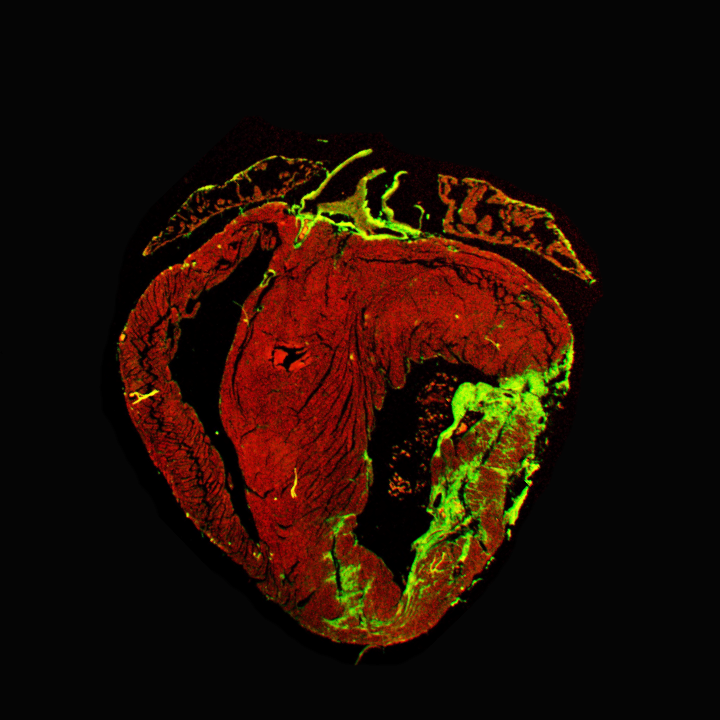
Role of ZEB2 transcription factor in oxidative stress response
Click for more
Role of ZEB2 transcription factor in oxidative stress response
Project Description
During our latest research, we identified the Zinc Finger E-box Binding Homeobox2 (ZEB2) transcription factor as an essential player during cardiac repair. We showed that ZEB2 is increased in stressed cardiomyocytes and induces cardioprotective crosstalk between cardiomyocytes and endothelial cells to enhance angiogenesis after MI. Cardiomyocyte-specific deletion of ZEB2 (Zeb2 cKO) resulted in impaired cardiac contractility and infarct healing after MI, while cardiomyocyte-specific ZEB2 overexpression (cardiomyocyte-specific ZEB2 transgenic – Zeb2 cTg) improved cardiomyocyte survival and cardiac function. We identified Thymosin b4 (TMSB4) and Prothymosin a (PTMA) as the main paracrine factors released from cardiomyocytes that stimulate angiogenesis by enhancing endothelial cell migration in vitro and in vivo (Gladka et al. 2021 Nat. Comm). Recently we linked ZEB2 to oxidative stress showing that ZEB2 overexpression protects the heart from ischemic damage. Therefore, we aim to identify and validate novel therapeutic targets downstream of ZEB2 to protect the heart from ischemia-induced dysfunction, and coax these into new therapies that will improve the survival and quality of life of IHD patients.
Funded by Dutch Heart Foundation
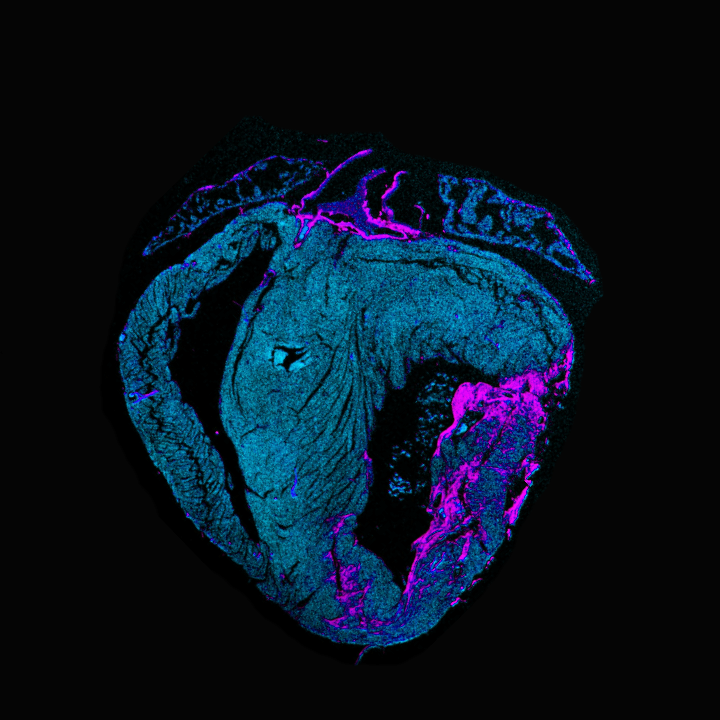
Function of long non-coding RNA Zeb2 opposite strand (Zeb2os) in cardiac ischemia
Click for more
Function of long non-coding RNA Zeb2 opposite strand (Zeb2os) in cardiac ischemia
Project Description
Our preliminary data show the critical function of the long non-coding RNA Zeb2 opposite strand (Zeb2os in mice) or (ZEB2-AS1 in humans) in the injured heart. Zeb2os overlaps and is antisense to ZEB2 at the 5′ splice site of an intron within the 5′ UTR. Previous studies have shown that Zeb2os prevents splicing of the intron-containing internal ribosome entry site (IRES), increasing the translation of the ZEB2 transcript. Zeb2os has pro-angiogenic and anti-apoptotic functions in different organs. However, its role in the heart still has to be determined.
To explore the potential protective role of Zeb2os in the injured heart, we delivered Zeb2os to mice undergoing sham or ischemia-reperfusion (IR) surgery via AAV9-mediated intramyocardial delivery. While the increase in Zeb2os did not affect cardiac outcomes at baseline, it worsened cardiac function in mice subjected to cardiac ischemia. Intriguingly, we observed that Zeb2os overexpression led to decreased ZEB2 protein levels. Conversely, in vitro Zeb2os knockdown increased ZEB2 protein expression. These findings suggest that Zeb2os regulates cardiac ZEB2 expression by reducing, rather than enhancing, the protein levels.
From a therapeutic perspective, our ongoing research involves in vivo Zeb2os knockdown in mice undergoing sham or IR surgery. Furthermore, we are dissecting the molecular mechanisms governing the Zeb2os-ZEB2 regulatory axis. These investigations hold promise for developing new and improved therapies for ischemic heart diseases.
Funded by Dutch Heart Foundation
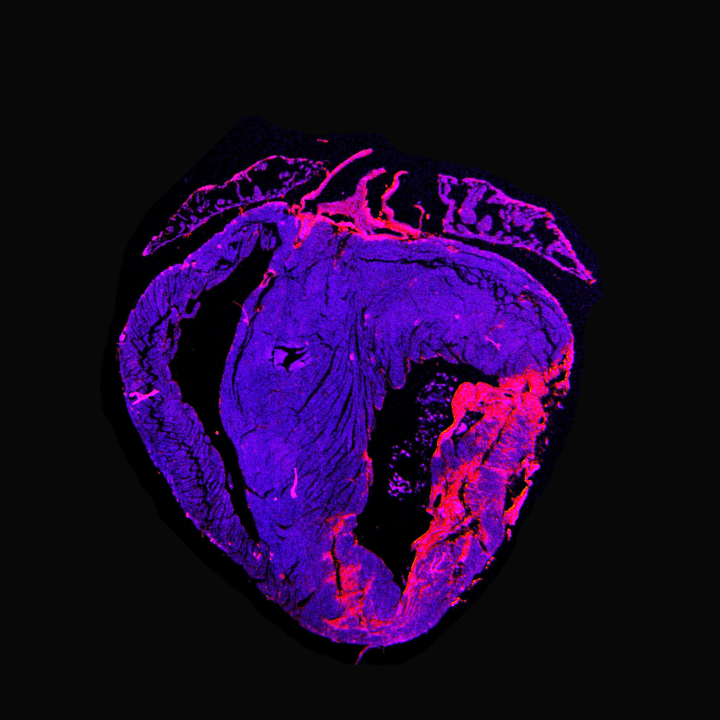
Target discovery with single-nuclei RNA sequencing
Click for more
Target discovery with single-nuclei RNA sequencing
Project Description
In this project, we aim to identify novel therapeutical targets that could be used for future purposes of cardiac gene therapy. We focused on examining adult hearts collected from human donors with post-mortem time below 5 hours. Samples of left ventricles were molecularly and morphologically characterized for the presence of stress, cardiac damage, and cardiac fibrosis. These results were cross-referenced with the donors’ medical history to classify each specimen as “control” or “diseased.” Due to the adult tissue’s complexity and the specimen’s high-fiber content, we developed an improved nuclei isolation protocol. Eventually, we successfully sequenced an average of 8.000 nuclei per sample with a gene coverage of approximately 800 genes per nucleus, identifying, via unsupervised cluster analysis, 17 different cell populations.
First, we focused on cardiomyocytes, identifying over 500 genes differentially expressed in diseased cells compared to healthy controls. Among these genes, we discovered several novel target genes whose expression is increased only in diseased cardiomyocytes. We are currently investigating the molecular effects of the gain-and-loss function of these genes in healthy iPS-derived cardiomyocytes and human-derived myocardial tissue slices to understand better their role in the pathophysiology of the adult human heart. From a therapeutic perspective, our ongoing research holds promises for developing new and improved intervention therapies to promote cardiac regeneration.
Funded by Amsterdam Cardiovascular Sciences
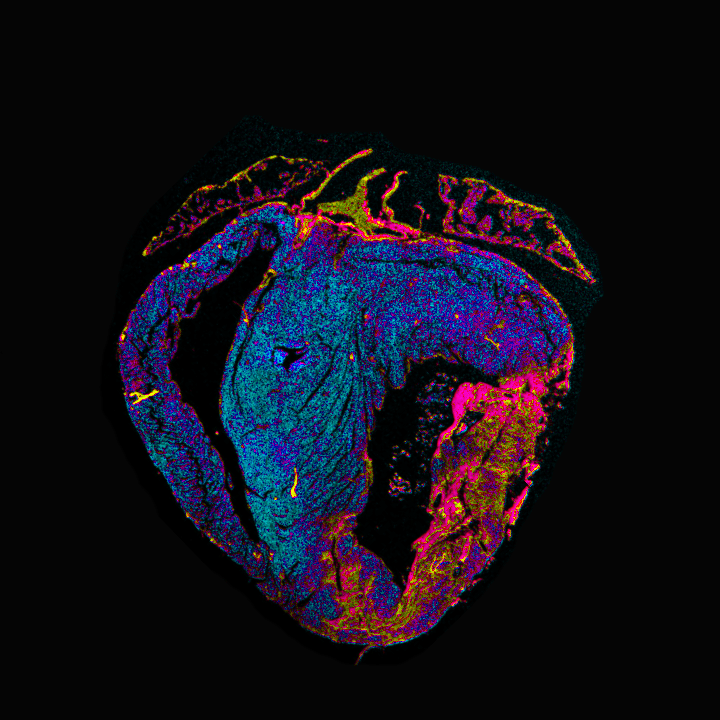
Developing state-of-the-art culturing systems for myocardial slice research
Click for more
Developing state-of-the-art culturing systems for myocardial slice research
Project Description
Deriving living tissue slices from human and animal organs is a technique widely used in several research fields for decades. In cardiac research, the first studies conducted in tissue slices derived from explanted hearts date back to the 1970s. However, due to the unique physiological needs of the heart, myocardial tissue slices degrade over time when kept in culture for days or months. Unlike other cell types, cardiomyocytes require constant mechanical and electrical stimulation to remain viable.
Recently, a few culturing systems have been introduced to recreate the physiological conditions in which myocardial slices remain viable. However, these systems are costly and allow simultaneous culturing of a very limited number of tissue slices. The low number of slices remains a significant limitation of these set-ups since biomedical research often requires a high number of technical and biological replicates for statistical reasons.
In collaboration with the University of Twente, we recently started developing a new, budget-friendly, high-throughput culturing system for myocardial tissue slices. We will use it for multiple applications, including fundamental research studies, drug testing, gene therapy testing, and disease modeling.
Myocardial tissue slices represent an intermediate-complexity model between laboratory models and human clinical trials that could be pivotal in advancing therapeutic strategies to treat diseased human hearts.
Collaborative project with Twente University
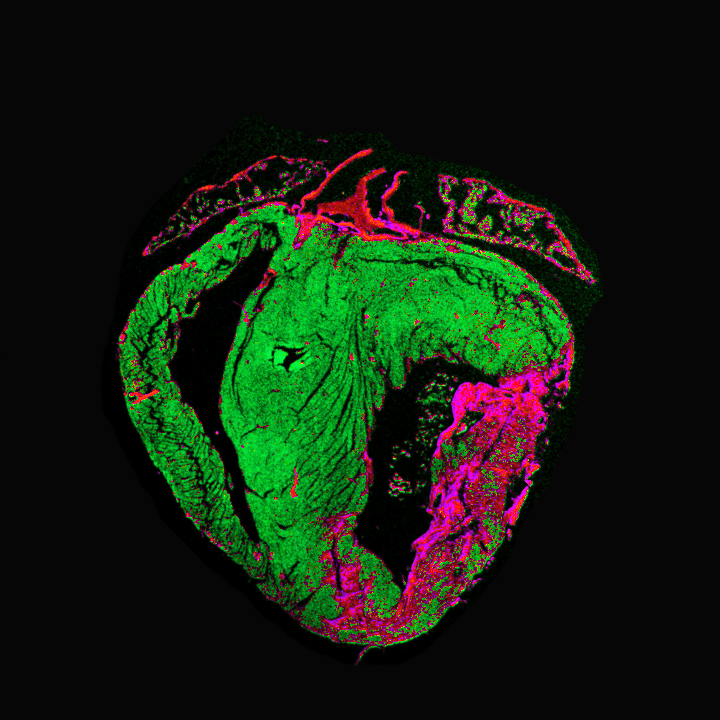
Cardiac slices to study cardioprotection during ischemic injury
Click for more
Cardiac slices to study cardioprotection during ischemic injury
Project Description
Heart slice culture is an emerging technology that solves many problems related to conventional myocardial culture systems. Heart slices have recently been used in several studies, demonstrating their ability to maintain the adult phenotype in a multi-cell environment for long periods. We propose testing gene therapy in living myocardial slices from fetal and adult human hearts. This collaborative project aims to use a translational approach by utilizing regenerative and non-regenerative human ex vivo models to study the onset, development, and repair of ischemic heart failure as it may occur in patients.
The group of our collaborator, Dr. Smits in LUMC, is developing human fetal cardiac slices as a regenerative platform to study cardiac regeneration. Similarly, our group in AUMC uses adult human cardiac slices to test advanced gene therapy approaches to promote long-term cardioprotection from cardiac ischemic injury.
We aim to combine these complementary models to study time-dependent alterations post-ischemic injury and identify potential therapeutic targets suitable for gene therapy applications.
Collaborative project with Dr Smits (Leiden UMC) – Funded by Rembrandt Institute
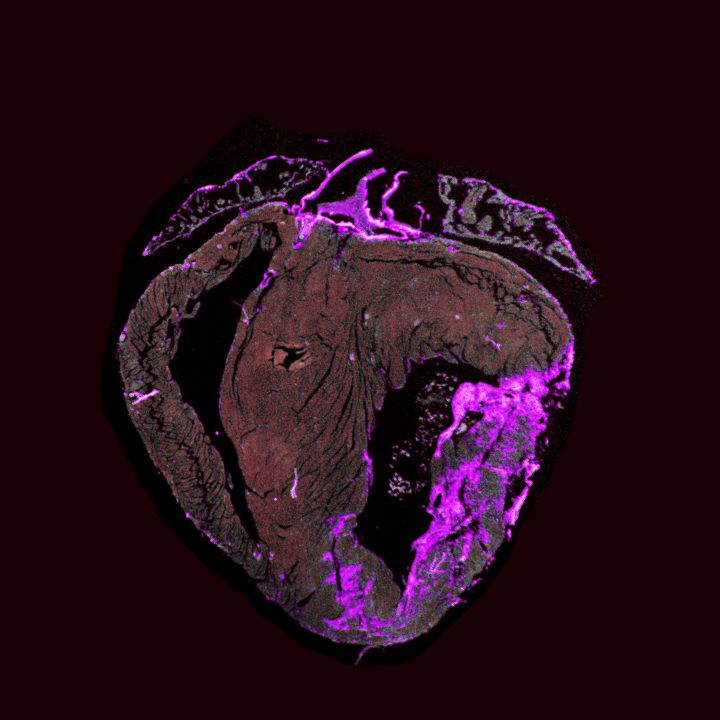
Gene therapy to stimulate cardiac regeneration
Click for more
Gene therapy to stimulate cardiac regeneration
Project Description
In this collaborative project with Professor Christoffels, we aim to drive cardiac regeneration through gene therapy approaches. We expect to advance our understanding of cardiac ischemia and congenital heart disease, leading to nowel future treatments. We use various strategies that combine in vitro, ex vivo, and in vivo disease models.
Our primary objective is to stimulate cardiac regeneration by fostering cardiomyocyte proliferation and angiogenesis while mitigating the detrimental effects of oxidative stress responses and effectively hampering apoptosis. We harness the power of AAV-mediated gene transfer to achieve these goals, employing a diverse range of potential factors to orchestrate this regenerative process.
Collaborative project with Prof. Christoffels (Amsterdam UMC)
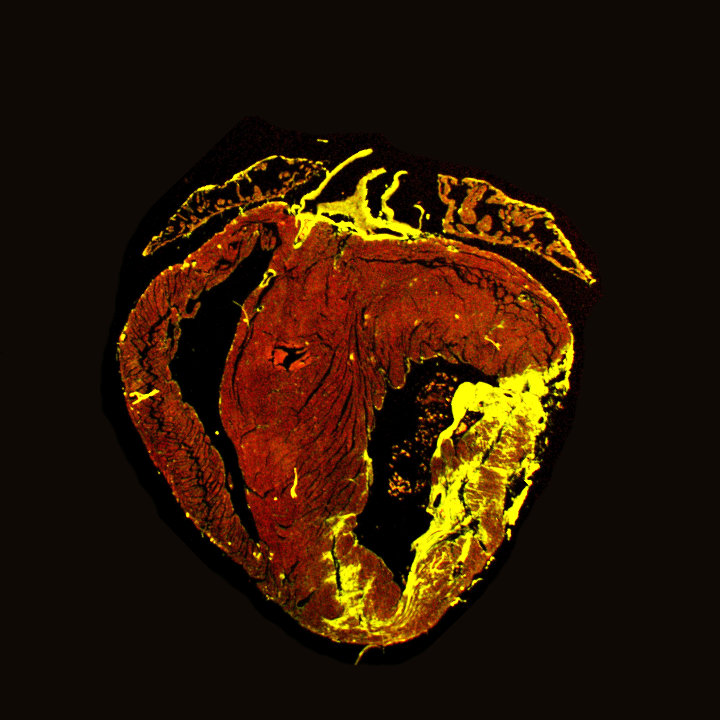
Novel gene therapies for Hypertrophic Cardiomyopathy
Click for more
Novel gene therapies for Hypertrophic Cardiomyopathy
Project Description
In this Double Dose mini-consortium, we research hypertrophic cardiomyopathy (HCM), the most common genetic heart disease. HCM is a progressive and chronic disease that can cause heart failure-like symptoms and confers an increased risk of life-threatening arrhythmias. There is only one disease-specific compound to treat HCM, Mavacamten, a cardiac myosin inhibitor that the FDA approved in April 2022. However, to date, no treatment is available that prevents or delays the disease process, which is partly because of a lack of understanding of the disease process.
HCM is not only a disease of cardiomyocytes, but also other cell types, such as fibroblasts and endothelial cells, contribute to the disease process. It was previously reported that microvascular dysfunction and perfusion deficits are seen in patients (Olivotto I et al. JACC 2011, Petersen SE et al. Circ 2007). Hence, we aim to test previously identified cardioprotective and pro-angiogenic gene therapies in settings of hypertrophic cardiomyopathy caused by MYBPC3 pathologic variants.
Collaborative project with: Dr Kuster (Amsterdam UMC) and Dr van Steenbeek (Utrecht UMC) – Funded by DCVA Double Dose Consortium
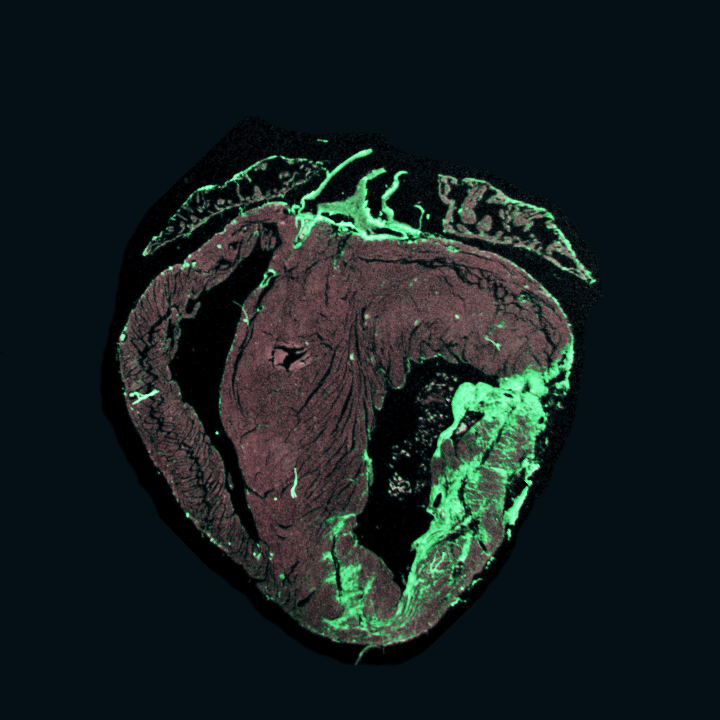
Amniotic progenitor secretome for cardioprotective therapies
Click for more
Amniotic progenitor secretome for cardioprotective therapies
Project Description
AmnioSMART, launching in May 2024, is a cutting-edge interdisciplinary project supported by recent funding. Our international team of researchers from Italy, Portugal, Belgium, and the Netherlands aims to revolutionize regenerative medicine for cardiac care.
AmnioSMART’s core objective is to standardize a secretome formulation with powerful anti-inflammatory and cardioprotective properties, targeting acute and chronic myocardial injuries. To achieve this, we’re utilizing perinatal progenitor sources: mesenchymal stromal cells from the amniotic membrane (hAMSC) and amniotic fluid (hAFSC).
Our innovation extends to creating efficient delivery systems for the optimal secretome. This project integrates regenerative medicine, tissue engineering, bioprinting, hiPSC technology, human cardiac slice culture, single nuclei RNA sequencing, and pharmacokinetic modeling for precise paracrine therapeutics administration. We’re pushing beyond the state-of-the-art to achieve ambitious yet attainable goals.
Consortium project with: Prof. Bollini (Genova, Italy), Prof. Parolini (Rome, Italy), Prof. Reis (Braga, Portugal), Prof. Sampaolesi (Leuven, Belgium) – Funded by ERA4Health CARDINNOV program
Publications
Caliandro R, Husetić A, Ligtermoet ML, Hanemaaijer-van der Veer J, van Duijvenboden K, Zentilin L, Clerkx M, Giacca M, Oostra RJ, van den Hoff MJB, Christoffels VM, Gladka MM AAV6-based ZEB2 delivery promotes cardiomyocyte dedifferentiation in adult human myocardium. Cardiovascular Research, 2025 Oct10:cvaf190 [IF2025=13.08]
https://doi.org/10.1093/cvr/cvaf190
Chiva-Blanch G, Liehn E, Andreadou I, Barc J, Brundel BJJM, Davidson SM, Elliott P, Evans PC, Giricz Z, Gladka MM, Gollmann-Tepeköylü C, Kleinbongard P, Krieg T, Linde C, Lüscher TF, Guzik T, Maguy A, McDonagh T, Paillard M, Parma R, Pesce M, Pompilio G, Rubini M, Streckfuss-Bömeke K, Thielmann M, Tocchetti CG, Van Linthout S, Vardas P, Vranckx P, Wojta J, Perrino C. Importance of basic science and research training for the future generation of cardiologists. European Heart Journal, 2025 Sep 25:ehaf738 [IF2025=39.3]
https://doi.org/10.1093/eurheartj/ehaf738
PR Goody, D Christmann, D Goody, S Hildebrand, H Billig, D Nehl, R Chennupati, M. Gladka, K Wilhelm‑Jüngling, S Uchida, S Iris‑Bibli, JB Moore IV, N Hamdani, F Paneni, SS Pullamsetti, S Zimmer, F Jansen, F Bakhtiary, E Aikawa, A Pfeifer, G Nickenig, MR Hosen. Calcific aortic valve disease augments vesicular microRNA-145-5p to regulate the calcification of valvular interstitial cells via cellular crosstalk. Basic Research in Cardiology, 2025 Aug 22;120(5):991-1010 [IF2025=8]
https://doi.org/10.1007/s00395-025-01133-w
Liangyu Hu, Alexia van Rinsum, Rocco Caliandro, Xi Qi, Shuxiu Fan, Xinyi Jiang, Jur Massop, Melissa Bekkenkamp-Grovenstein, Christiane Ott, Tilman Grune, Melissa A. E. van de Wal, Werner J. H. Koopman, Marcel Giesbers, Monika Gladka, Jaap Keijer & Deli Zhang. A robust method for assessing mitochondrial function in healthy and diseased frozen cardiac tissue. Communication Biology, 2025 Aug 19;8:1249 [IF2025=5.2]
https://doi.org/10.1038/s42003-025-08608-5
Rocco Caliandro, Azra Husetić, Merel L Ligtermoet, Arie R Boender, Lorena Zentilin, Gerard J J Boink, Mauro Giacca, Monika M Gladka. Living myocardial slices as a model for testing cardiac pro-reparative gene therapies. Molecular Therapy, 2025 Jul2;33(7):2990-2996 [IF2024=12.4]
https://doi.org/10.1016/j.ymthe.2025.03.033
AmnioSMART Consortium: Sveva Bollini, Ornella Parolini, Rui L Reis, Ricardo A Pires, Maurilio Sampaolesi, Monika M Gladka. Optimizing the human perinatal progenitor cell secretome for future paracrine therapy to counteract cardiac disease with AmnioSMART: an ERA4Health-funded project. European Heart Journal, 2024 Oct 16:ehae550 [IF2023=37.6]
Gladka MM, Kohela A, de Leeuw AE, Molenaar B, Versteeg D, Kooijman L, van Geldorp M, van Ham WB, Caliandro R, Haigh JJ, van Veen TAB, van Rooij E. Hypoxia-responsive zinc finger E-box-binding homeobox 2 (ZEB2) regulates a network of calcium-handling genes in the injured heart. Cardiovascular Research, 2024 Sep 23:cvae163 [IF2023=10.2]
2023
Gladka MM, van der Velden J. Scientists on the spot: Relaxing the heart in hypertrophic cardiomyopathy. Cardiovascular Research, 2023 Apr 26;cvac049 [IF2022=14.239]
Bollini S, Gladka MM, Smits AM. Editorial: Straight from the heart: Novel insights and future perspectives for cardiac repair. Frontiers in Cardiovascular Medicine, 2023 Feb 14;10:1149626 [IF2021=6.05]
2022
Gladka MM*, Johansen AKZ*, van Kampen SJ, Peters MMC, Molenaar B, Versteg D, Kooijman L, Zentilin L, Giacca M, van Rooij E. Thymosin β4 and prothymosin α promote cardiac regeneration post-ischemic injury in mice. Cardiovascular Research, 2022 Sep 20;cvac155 [IF2021=14.239]
Gladka MM, Baker AH. Cutting a path to effective delivery of genome engineering machinery. Cardiovascular Research, 2022 May 6;118(6):e42-e44 [IF2021=14.239]
Gladka MM, Christoffels VM. Studying the role of chromatin organization in cardiovascular disease: future perspectives. Cardiovascular Research, 2021 Nov 1;117(12):e156-e158 [IF2021=14.239] https://doi.org/10.1093/cvr/cvab319
Gladka MM, Pasterkamp G. Scientists on the Spot: Re-defining atherosclerosis through biobanks. Cardiovascular Research, 2021 Jul 7;117(8):e99-e100 [IF2021=14.239]
Gladka MM, Giacca M. Scientists on the Spot: Re-awakening the heart’s regenerative capacity. Cardiovascular Research, 2021 May 25;117(6):e79-e81 [IF2021=14.239]
Gladka MM, Baker AH. Jumping on base editing to repair the diseased cardiovascular system in vivo. Cardiovascular Research, 2021 Mar 21;117(4):e46-e48 [IF2021=14.239]
Gladka MM. Single-Cell RNA Sequencing of the Adult Mammalian Heart-State-of-the-Art and Future Perspectives. Current Heart Failure Reports, 2021 Apr;18(2):64-70 [IF2021=2.192]
Molenaar B*, Timmer LT*, Droog M, Perini I, Versteeg D, Kooijman L, Monshouwer-Kloots J, de Ruiter H, Gladka MM, van Rooij E. Single-cell transcriptomics following ischemic injury identifies a role for B2M in cardiac repair. Communications Biology, 2021 Jan 29;4(1):146 [IF2021=6.548]
Gladka MM, Kohela A, Molenaar B, Versteeg D, Kooijman L, Vos HR, Huibers MMH, Haigh JJ, Huylebroeck D, Giacca M, van Rooij E. Cardiomyocytes stimulate angiogenesis after ischemic injury in a ZEB2-dependent manner. Nature Communication, 2021 Jan 4;(1):84 [IF2021=14.919]
Gladka MM, Stellos K. Scientists on the Spot: RNA modification in atherosclerosis. Cardiovascular Research, 2021 Jan 1;117(1):e9 [IF2021=14.239]
Gladka MM, Maack C. The endothelium as Achilles’ heel in COVID-19 patients. Cardiovascular Research, 2020 Dec 1;116(14):e195-e197 [IF2021=14.239]
M.F. Hoes, A.S.J.M. te Riele, M.M. Gladka, B.D. Westenbrink, G.P.J. van Hout, M.M.G. van den Hoogenhof, A. Ghigo, S. Bollini, N.H. Purcell, I. Kardys, D.W.D. Kuster. Position paper: Empowering the next generation of cardiovascular researchers. Netherlands Heart Journal, 2020 Aug;28(Supp 1):25-30 [IF2021=2.38]
Vigil-Garcia M*, Demkes CJ*, Eding JEC, Versteeg D, de Ruiter H, Perini I, Gladka MM, Asselbergs FW, Vink A, Harakalova M, Bossu A, van Veen TAB, Boogerd CJ, van Rooij E. Gene expression profiling of hypertrophic and failing cardiomyocytes identifies new players in heart failure. Cardiovascular Research, 2020 Jul 27:cvaa233 [IF2021=14.239]
Gladka MM Cellular communication in a “virtual lab”: going beyond the classical ligand-receptor interaction. 2020; Cardiovascular Research, 2020 Jun 1;116(7):67-e69 [IF2021=14.239]
Gladka MM*, Molenaar B*, de Ruiter H, van der Elst S, Tsui H, Versteeg D, Lacraz GPA, Huibers MMH, van Oudenaarden A, van Rooij E. Single-cell sequencing of the healthy and diseased heart reveals Ckap4 as a new modulator of fibroblasts activation. Circulation, 2018 Jul 10;138(2):166-180 [IF2021=39.918]
https://doi.org/10.1161/circulationaha.117.030742
Lacraz GPA*, Junker JF*, Gladka MM, Molenaar B, Scholman KT, Vigil Garcia M, Versteeg D, de Ruiter H, Vermunt MW, Creyghton MP, Huibers MMH, Nicolaas de Jonge N, van Oudenaarden A, van Rooij E. Tomo-seq identifies SOX9 as a key regulator of cardiac fibrosis during ischemic injury. Circulation, 2017 Oct 10;136(15):1396-1409 [IF2021=39.918]
https://doi.org/10.1161/circulationaha.117.027832
Gladka MM, van Rooij E. AntimiR-34a to enhance cardiac repair after ischemic injury. Circulation Research, 2015 Aug14;117(5):395-7 [IF2021=17.367]
https://doi.org/10.1161/circresaha.115.307066
Dirkx E*, Gladka MM*, Philippen LE, Armand AS, Kinet V, Leptidis S, El Azzouzi H, Salic K,Bourajjaj M, da Silva GJ, Olieslagers S, van der Nagel R, de Weger R, Bitsch N, Kisters N, Seyen S, Morikawa Y, Chanoine C, Heymans S, Volders PG, Thum T, Dimmeler S, Cserjesi P, Eschenhagen T, da Costa Martins PA, De Windt LJ. Nfat and miR-25 cooperate to reactivate the transcription factor Hand2 in heart failure. Nature Cell Biology, 2013 Nov;15(11):1282-93 [IF2016/2017=28.824]
https://doi.org/10.1038/ncb2866
Gladka MM, da Costa Martins, De Windt LJ. Small changes can make a big difference – MicroRNA regulation of cardiac hypertrophy. Journal of Molecular and Cellular Cardiology, 2012 Jan;52(1):74-82 [IF2021=5.00]
https://doi.org/10.1016/j.yjmcc.2011.09.015
Da Costa Martins PA*, Salic K*, Gladka MM, Armand AS, Leptidis S, el Azzouzi H, Hansen A, Coenen-de Roo CJ, Bierhuizen MF, van der Nagel R, van Kuik J, de Weger R, de Bruin A, Condorelli G, Arbones ML, Eschenhagen T, De Windt LJ. MicroRNA-199b targets the nuclear kinase Dyrk1a in an auto-amplification loop promoting calcineuring/NFAT signaling. Nature Cell Biology, 2010 Dec;12:1220-7 [IF2016/2017=28.824]
https://doi.org/10.1038/ncb2126
Gladka M, el Azzouzi H, De Windt LJ, da Costa Martins PA. Aquaporin 7: the glycerol aquaeductus in the heart. Cardiovascular Research, 2009 Jul;83 (1):3-4 [IF2021=14.239]
https://doi.org/10.1093/cvr/cvp147
Da Costa Martins PA, Bourajjaj M, Gladka M, Kortland M, van Oort RJ, Pinto YM, Molkentin JD, De Windt LJ. Conditional dicer gene deletion in the postnatal myocardium provokes spontaneous cardiac remodeling. Circulation, 2008 Oct7;118:1567-76 [IF2021=39.918]
https://doi.org/10.1161/circulationaha.108.769984
Gallery

Cardiovascular Mater Track practical course by Rocco and Azra, October 2024, Amsterdam, The Netherlands

Merel’s Master Graduation Ceremony, October 2024, Utrecht, The Netherlands

1st Poster Prize for Rocco at the 8th DCVA Translational Cardiovascular Research Meeting, June 2024, Utrecht, The Netherlands

Gala dinner at ISHR meeting, June 2024, Toulouse, France

ISHR meeting, June 2024, Toulouse, France
Poster session at Cardioascona conference, May 2024, Ascona, Switzerland

Dutch-German Joint Meeting (DGJM), March 2024, Groningen, The Netherlands

Preparation of myocardial slices from human heart, January 2024, Amsterdam, The Netherlands

Azra dissecting human heart, January 2024, Amsterdam, The Netherlands

Christmas party, December 2023, Amsterdam, The Netherlands

ESC Working Groups on Cellular Biology of the Heart & Myocardial Function, November 2023, Naples, Italy

European Society of Cardiology Congress, August 2023, Amsterdam, The Netherlands

7th DCVA-NLHI Translational Cardiovascular Research Meeting, July 2023, Utrecht, The Netherlands
Poster Session, 7th DCVA-NLHI Translational Cardiovascular Research Meeting, July 2023, Utrecht, The Netherlands

Rocco and Alex dissecting mouse, May 2023, Amsterdam, The Netherlands
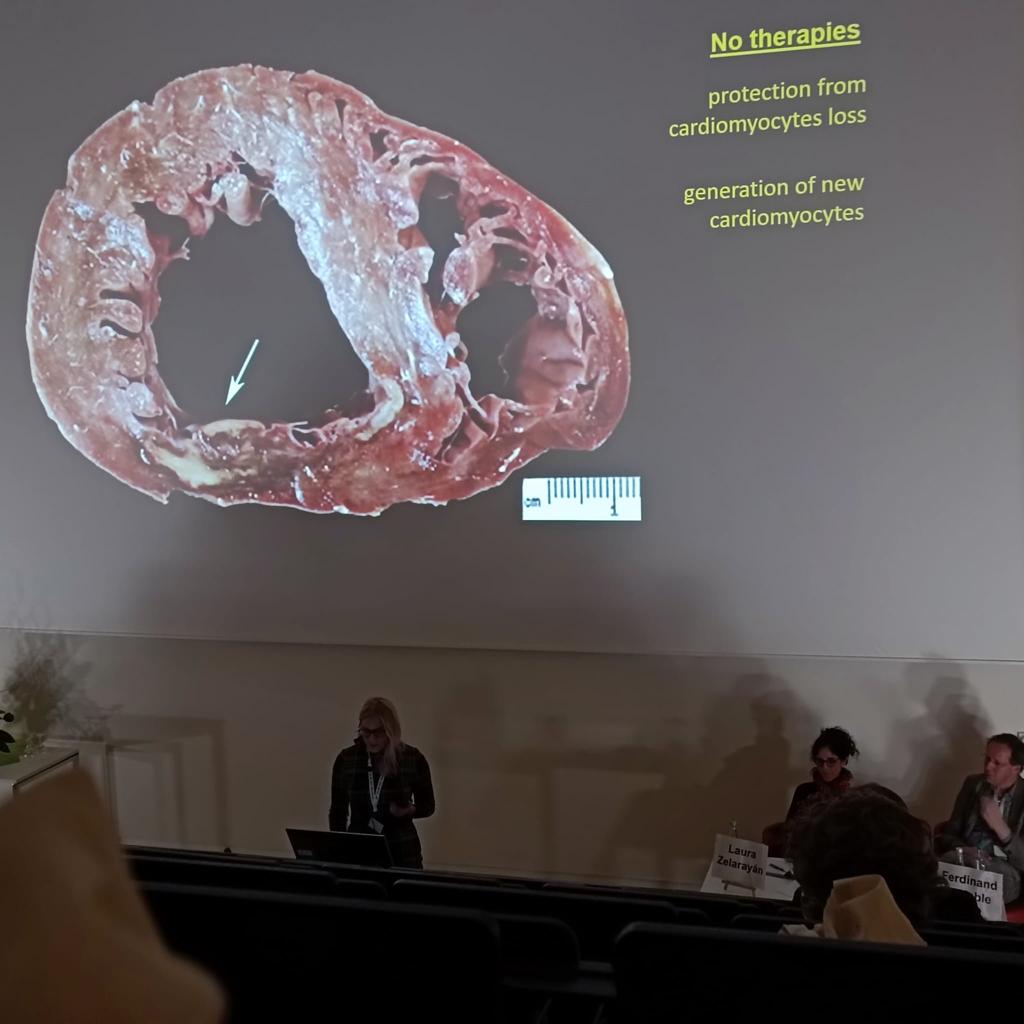
Dutch-German Joint Meeting (DGJM), March 2023, Würzburg, Germany
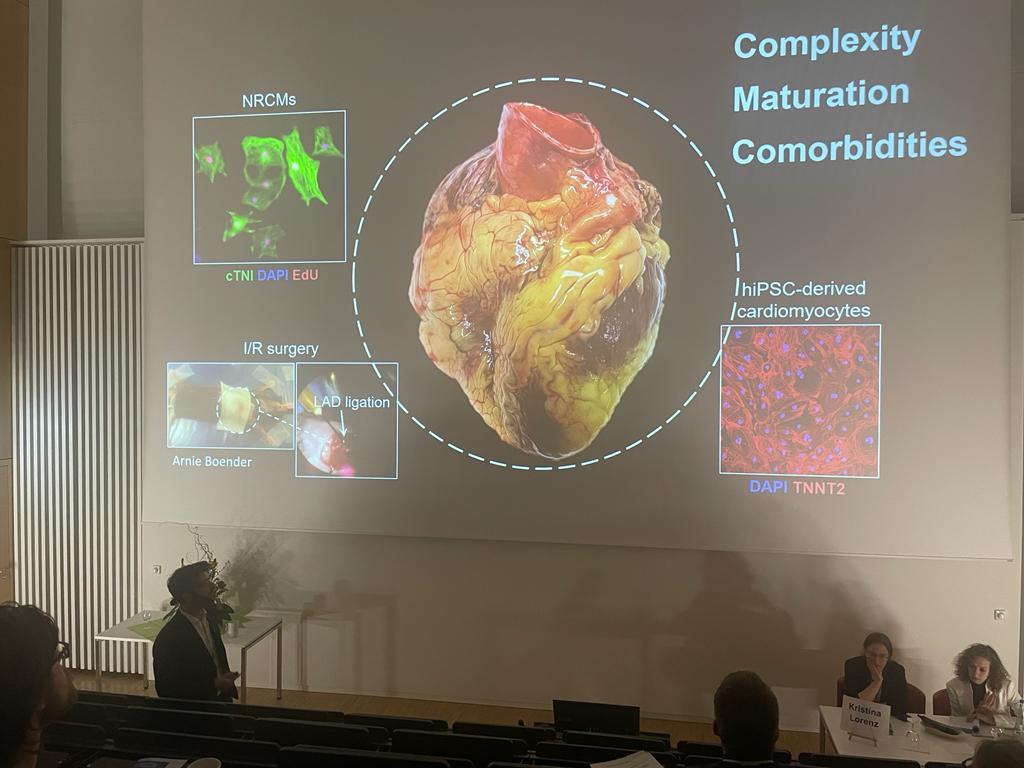
Young Investigator Competition, Dutch-German Joint Meeting (DGJM), March 2023, Würzburg, Germany
Imaging proliferation in the heart, November 2022, Amsterdam, The Netherlands

Preparation of myocardial slices from a pig heart, September 2022, Amsterdam, The Netherlands
Finalizing in vivo studies, July 2022 (Rocco, Alex, Arnie), Amsterdam, The Netherlands

Amsterdam Cardiovascular Sciences Symposium, September 2022, Amsterdam, The Netherlands

6th DCVA Translational Cardiovascular Research Meeting, June 2022, Utrecht, The Netherlands
Poster Session, Weinstein Cardiovascular Development and Regeneration Conference 2022, Marseille, France

Frontiers in Cardiovascular Biomedicine (FCVB) 2022
Budapest, Hungary

Young@Heart Spring event 2022, Utrecht, The Netherlands
Contact
Monika Gladka, PhD
Associate Professor, Principal Investigator
Department of Medical Biology,
Amsterdam University Medical Centers,
Amsterdam Cardiovascular Sciences,
Meibergdreef 15, 1105 AZ Amsterdam, Room L2-108-1, Amsterdam, The Netherlands
Phone: +3120 566 7696
Mobile: +316 5163 2185
Email: m.m.gladka@amsterdamumc.nl